#drafting protocol for clinical trial
Explore tagged Tumblr posts
Text

3 Tips on Protocol Writing from an Investigator’s Perspective
#protocol writing in clinical research#protocol writing guidelines#clinical trial protocol writing#drafting protocol for clinical trial#writing protocols for clinical trials
0 notes
Text
Vishwang Desai’s Thoughts on Investment Potential and Legal Framework for Neuro-Tech in India
India stands at the cusp of a technological revolution with the emergence of neuro-tech and brain-computer interface (BCI) sectors. While the global neuro-tech market is projected to surge beyond $20 billion by 2026, Vishwang Desai strongly feels that India's participation remains nascent, hindered by a complex regulatory landscape, ethical dilemmas, and infrastructural inadequacies. For investors, the potential is evident, but the pathway is fraught with challenges that extend beyond mere capital infusion. In this context, legal professionals are increasingly required to navigate a labyrinth of laws governing data privacy, biomedical research, and technology transfers.
The Promise of Neuro-Tech: Immense Untapped Potential
The neuro-tech sector encapsulates devices and systems designed to interact with the human brain, ranging from non-invasive neuro-monitoring systems to invasive brain implants that control prosthetics. India's tech-savvy population and burgeoning healthcare sector provide fertile ground for growth. Government policies, such as the National Digital Health Mission (NDHM), have already set the stage for integrating health tech with AI and data analytics, creating a conducive environment for neuro-tech expansion.
However, India's current regulatory framework is relatively silent on neuro-tech-specific governance. The Medical Devices Rules, 2017, cover biomedical equipment but do not explicitly address neuro-tech or BCIs. Moreover, the Clinical Establishments (Registration and Regulation) Act, 2010, and the Drugs and Cosmetics Act, 1940, provide general guidelines but are ill-equipped to handle the nuanced risks associated with brain-computer interfaces. Legal professionals must therefore advise clients on the broader implications of data privacy under the Digital Personal Data Protection Act, 2023, particularly concerning the collection, processing, and transmission of neural data, which could potentially include biometric identifiers.
Legal and Security Challenges: Privacy, Data, and Ethics
One of the most contentious areas for neuro-tech development in India is data privacy. The Digital Personal Data Protection Act, 2023, outlines stringent norms for handling sensitive personal data, including health data and biometric information. For companies developing BCIs, the challenge lies in obtaining explicit consent, safeguarding data storage, and ensuring cross-border data transfer compliance. Legal professionals must meticulously draft data protection agreements, particularly considering that neuro-data can potentially reveal cognitive patterns and behavioral insights, raising ethical and privacy concerns.
Further, the Biomedical Research Regulation and Reporting System under the Indian Council of Medical Research (ICMR) stipulates guidelines for human trials involving neurological devices. The guidelines mandate robust informed consent protocols and data anonymization, which are crucial given that BCIs inherently interface with the brain, potentially exposing personal and proprietary neurological data. Failure to adhere to these guidelines may lead to severe liabilities under the Consumer Protection Act, 2019, particularly concerning defective products and negligent services.
Investment Roadblocks and Policy Gaps
While the neuro-tech sector in India presents lucrative opportunities, investment barriers persist. Intellectual property (IP) protection remains a critical concern. BCIs often involve proprietary algorithms and hardware systems that require patent protection. However, India’s patent regime, governed by the Patents Act, 1970, is yet to clearly define the scope of neuro-tech innovations, particularly in the realm of software embedded in medical devices. This legal ambiguity deters foreign investors, especially when juxtaposed with more comprehensive frameworks in jurisdictions such as the US and the EU.
Additionally, taxation policies for high-tech medical devices, including BCIs, remain cumbersome. The Goods and Services Tax (GST) rates applicable to medical devices are relatively high, impacting the cost structure for neuro-tech companies. Moreover, the absence of dedicated government incentives or subsidies for neuro-tech R&D further dissuades potential investors. Given these challenges, legal experts must advise clients on navigating tax exemptions, claiming R&D credits, and structuring cross-border investments to mitigate regulatory risks.
Conclusion: A Call for Legal and Regulatory Reforms
India’s neuro-tech sector is ripe for investment, but realizing its full potential requires targeted regulatory reforms. Policymakers, in the opinion of Vishwang Desai ,must consider implementing a comprehensive framework specific to neuro-tech and BCIs, integrating data privacy, biomedical ethics, and IP protection under a unified legislative framework. Legal professionals, particularly those specializing in health tech and data privacy, will play a crucial role in shaping the regulatory landscape, ensuring that India not only attracts foreign investments but also safeguards the cognitive rights and privacy of its citizens in an increasingly digitized world.
Read More of Our Blogs at: https://vishwangdesai.tumblr.com/
1 note
·
View note
Text
How AI-Powered Medical Writing Services Are Transforming Clinical Research

In the dynamic landscape of the life sciences industry, where precision is crucial, medical writing services have become a vital ally for companies navigating the complexities of regulatory requirements. This year, advancements in AI, data automation, and improved content organization are poised to transform how we approach medical documentation, making it more effective for regulatory submissions, transparency in clinical trials, and scientific communication.
For clinical research organizations (CROs), pharmaceutical sponsors, or biotech innovators, choosing the right medical writing services can be a game-changer. It can streamline timelines, ensure compliance, and ultimately lead to more successful outcomes. When you partner with the right professionals, you’re not just ticking boxes; you’re building trust and credibility in a field that relies heavily on precision and clarity.
The Evolution of Medical Writing: 2025 Industry Trends
The role of medical writers has progressed considerably. They now engage not only in the preparation of scientific documents but also in data analysis, the integration of AI technologies, and teamwork with different departments. Below are some of the factors responsible for the transformation:
Regulatory complexity Agencies like the FDA and EMA are demanding greater transparency and standardization in clinical study reports, clinical protocol development, and DSURs.
AI and automation Natural language generation (NLG) tools and AI-powered templates are streamlining clinical trial writing services, particularly for repetitive content such as risk-benefit analyses and summaries of product characteristics.
Globalization Multinational studies necessitate localized yet consistent documentation across geographies and languages.
Structured Content Management Systems (SCMS) These platforms now serve as the backbone for content reuse, audit trails, and version control.
The Strategic Role of Medical Writing Services
These days, regulatory and medical writing services are about much more than just checking grammar and style. Writers act as trusted experts, turning complicated clinical data into clear, submission-ready documents. Key services include:
Clinical Trial Writing Services From phase I to IV, writers develop essential documents, including:
Clinical Study Reports (CSRs)
Investigator Brochures
Informed Consent Forms
Narratives and interim reports
With AI-assisted analytics, medical writers can identify trends in trial data and craft evidence-based narratives that support regulatory strategy.
Clinical Protocol Writing & Development Precise and robust protocols are vital to trial success. Developing clinical protocols now involves close collaboration across multiple disciplines, including biostatistics, pharmacovigilance, and regulatory affairs. By utilizing AI platforms, writers can create content tailored to meet regulatory requirements and the specific details of various therapeutic areas. Whether you’re preparing a new protocol or amending an existing one, protocol development services ensures:
Consistency across endpoints and methodologies
Alignment with trial objectives and statistical plans
Streamlined communication between global stakeholders
DSUR Writing and Risk Management Annual Development Safety Update Reports (DSURs) are a regulatory necessity, yet time intensive. AI tools now extract safety data from structured databases and automate tabulations, leaving writers to focus on risk interpretation and mitigation strategy. Expert DSUR Writing helps sponsors meet ICH E2F standards efficiently and thoroughly.
AI and Automation: A New Era for Regulatory & Medical Writing Services
Artificial intelligence has evolved from being a mere experimental technology to becoming an integral part of medical writing services. Here’s how it’s reshaping the industry:
Automated Drafting with NLP AI engines trained on regulatory documents can now draft portions of Clinical Study Reports, protocols, and summaries, cutting writing time by up to 40%.
Data-Driven Insights Integrated with electronic data capture (EDC) systems and CTMS, AI tools help writers spot inconsistencies or anomalies in trial data before they become compliance risks.
Structured Content Management System SCMS platforms enable the reuse of validated content blocks across multiple documents. For instance, adverse event descriptions or investigational product details can be automatically populated across DSURs, CSRs, and protocols. It reduces errors and shortens review cycles.
ACL Digital Life Sciences highlights how SCMS adoption has enhanced document quality and traceability, which is especially critical in regulatory audits.
Why Choose a Professional Medical Writing Services Company?
Not all service providers are the same, especially when it comes to medical writing. A professional medical writing services company combines a deep knowledge of various therapeutic areas, a firm grasp of technology, and a solid understanding of regulatory requirements. Here are some key offerings from top-tier companies:
Multilingual, global documentation support
Cross-functional medical, regulatory, and statistical writing teams
AI-augmented writing platforms and SCMS integration
Regulatory knowledge across the US, EU, APAC, and emerging markets
Robust quality control workflows for submission readiness
The end goal? Accelerate approvals, reduce rework, and enhance data integrity.
Real-World Applications: How Leading Sponsors Benefit
Faster Submissions with AI-Augmented Protocols A mid-size oncology sponsor partnered with an AI-enabled writing team to develop protocols for a multi-site Phase II trial. By using a structured content management system, they reduced protocol development time by 45%, with zero major revisions from the Institutional Review Board (IRB).
Improved DSUR Writing Accuracy A top 20 pharmaceutical company utilized automated data extraction for DSURs across five compounds. The medical writing team manually tailored risk assessments and conclusions, reducing submission errors and the time to finalize by 30%.
Streamlined Global Clinical Trial Writing Services A CRO managing trials in 12 countries leveraged centralized writing hubs and SCMS tools to ensure consistent clinical study reports and clinical protocol writing, improving compliance across diverse regulatory agencies.
Looking Ahead: The Future of Medical Writing Services
As artificial intelligence continues to develop, the expectations of sponsors, regulators, and patients will also evolve. In the coming years, we can anticipate greater implementation of:
Predictive analytics in protocol planning
Real-world data integration into study documents
Voice-assisted writing tools
Blockchain-enabled traceability in document development
Technology’s significance eventually hinges on the users behind it. Human skills are indispensable for analyzing data, providing context, and navigating complexities, particularly in areas related to regulatory and safety communication.
Final Thoughts
Currently, the tightly regulated clinical environment has led medical writing services to evolve from mere support functions to essential partners in achieving success. Whether you are crafting clinical protocols, preparing Development Safety Update Reports (DSURs), or managing extensive global clinical trial writing, integrating advanced technologies like AI and automation with skilled medical writers can provide significant advantages.
Collaborating with a professional medical writing services provider that utilizes cutting-edge tools — such as a structured content management system — helps ensure precision, compliance, and a quicker route to market. Get in touch with us and explore how AI-enhanced regulatory and medical writing Services can refine your clinical research process. Whether you require protocol development, DSUR creation, or submission-ready Clinical Study Reports (CSRs), our team of experts is ready to support you every step of the way.
Contact us at [email protected] to explore how we can enhance your workplace transformation.
This blog was originally published on the website www.acldigital.com
0 notes
Text
Decoding Clinical Research Protocols: From Study Design to Data Analysis
What Is a Clinical Research Protocol?
A clinical research protocol is a detailed plan that outlines how a clinical trial will be conducted. It includes the study’s rationale, methodology, objectives, eligibility criteria, data collection methods, statistical analysis plans, and ethical considerations.
Think of it as the blueprint for a building. Just as architects plan every room, door, and window before construction, researchers draft a protocol to ensure every aspect of a clinical trial is accounted for before it begins.
The Human Side of Protocols
While protocols might seem technical on the surface, they are deeply human documents. Why? Because every decision within a protocol—from inclusion criteria to data monitoring—is ultimately about protecting human participants and ensuring their rights and well-being are prioritized.
Key Components of a Clinical Research Protocol
Let’s break down the core parts of Decoding Clinical Research Protocols: From Study Design to Data Analysis:
1. Study Design
This section answers the “how” of the research:
Objective: What is the study aiming to find out?
Type of Study: Is it observational or interventional? Randomized or non-randomized?
Sample Size: How many participants are needed to get reliable results?
Duration: How long will the study take?
2. Eligibility Criteria
These define who can or cannot participate in the study.
Inclusion Criteria: Age, gender, diagnosis, treatment history, etc.
Exclusion Criteria: Factors that may interfere with the results or participant safety.
3. Intervention and Treatment Plan
Describes the treatment(s) being tested.
Includes dosage, administration route, and frequency.
Defines control or placebo use, if applicable.
4. Data Collection and Management
Specifies what data will be collected and at what intervals.
Clarifies how data will be stored and protected (ensuring confidentiality).
Often includes tools like Case Report Forms (CRFs) or electronic systems.
5. Statistical Analysis
This is where data turns into meaningful results:
Primary and Secondary Endpoints: Outcomes the study will measure.
Statistical Methods: How will the results be analyzed? Will it use regression models, survival analysis, etc.?
Handling Missing Data: How will incomplete data be addressed?
Importance of Ethical Oversight
In the process of Decoding Clinical Research Protocols: From Study Design to Data Analysis, ethical oversight remains a cornerstone. Institutional Review Boards (IRBs) or Ethics Committees review the protocol before any participant is enrolled.
This ensures:
Informed consent is properly obtained.
Participant risks are minimized.
Benefits outweigh potential harms.
Why Protocols Must Be Clear and Robust
A poorly written protocol can lead to confusion, safety issues, regulatory delays, or even study termination. A well-written protocol, on the other hand, improves:
Data reliability
Regulatory compliance
Participant safety
Study reproducibility
Real-World Applications and Lessons Learned
In recent years, with the rise of decentralized trials and wearable devices, clinical protocols have had to adapt. Protocols today often include provisions for remote monitoring, virtual visits, and real-time data sharing—all of which demand an even more thorough planning process.
From the COVID-19 vaccine trials to cancer research innovations, effective protocols have accelerated life-saving discoveries without compromising on ethics or quality.
The Role of the Clinical Research Professional
The role of clinical research professionals in developing, managing, and executing protocols is essential. Whether it's a clinical research coordinator ensuring accurate data capture or a biostatistician analyzing the final outcomes, every member of the team contributes to the success of the protocol.
Summary
Decoding Clinical Research Protocols: From Study Design to Data Analysis is more than just a technical process—it’s a blend of science, ethics, and humanity. Understanding protocols equips us to ask better questions, design better studies, and deliver better healthcare solutions. Whether you're a budding researcher or a curious healthcare professional, appreciating the art and science behind clinical research protocols is your first step toward impactful contributions in medicine.
Key Takeaways
Clinical research protocols are the foundation of every clinical study.
Protocols cover study design, participant selection, interventions, data collection, and statistical analysis.
Ethical oversight ensures participant rights and safety.
Clear, robust protocols reduce errors and improve trial efficiency.
The human element remains at the heart of every decision in a protocol.
0 notes
Text
Expert Thesis and Dissertation Support for Medical & Allied Health Students
At Rehoboth Academic Services, we specialize in providing end-to-end thesis support for medical students, including MD, MS, Nursing, Physiotherapy, and other allied health disciplines. Whether you're preparing a complex research proposal or analyzing quantitative data, our team of medical and allied health research experts is here to guide you every step of the way. The journey starts with thesis proposal writing, a critical foundation of any successful research study. Our team collaborates with you to understand your research goals, identify gaps, and create a strong theoretical framework that aligns with your university’s standards. If you're struggling with developing your concept note or facing challenges in organizing your ideas, we offer medical research proposal help tailored to your specialization. Whether it's clinical research, healthcare policy, or physiotherapy intervention, we ensure your proposal is research-worthy and ready for ethical review. For nursing graduates and postgraduates, our specialized service includes protocol development for nursing students. We assist in structuring a scientifically sound research protocol that meets both institutional and academic requirements. Determining the right sample size can be overwhelming, especially in quantitative studies. That’s why we offer sample size calculation support using statistical tools and validated methodologies to ensure the reliability and validity of your research outcomes. Once your data is ready, our statistical analysis for medical research helps you make sense of it all. Our biostatistics team works with tools like SPSS, R, and Excel to perform both descriptive and inferential analysis, tailored to your research objectives.
Postgraduate scholars in various disciplines also benefit from our biostatistics for postgraduate students service. Whether you're conducting a cross-sectional study, clinical trial, or longitudinal analysis, our experts provide personalized statistical insights and visualizations. In addition, we offer proposal drafting and revision services for students who already have a research idea but need help structuring it. We revise based on your feedback and academic feedback to make sure your submission is polished and impactful. Our dissertation help for allied health students covers everything from literature review, methodology design, to final proofreading. We ensure your work stands out with clear arguments, error-free language, and proper formatting. With Rehoboth, you’re not just getting academic support—you’re gaining a reliable research partner who understands the rigors of medical and allied health education.
#thesis#proposal writing#medical research#nursing students#statistical analysis#dissertation help#allied health#Postgraduate Students#Thesis Support for Medical Students#Health Research Experts
0 notes
Text
Teccro: A Top 10 Contract Research Organizations in India
TECCRO’s Comprehensive Medical Writing Services: Elevating Clinical Research with Precision and Expertise
At TECCRO (The Esthetic Clinics Clinical Research Organization), medical writing is a cornerstone of our clinical research services, ensuring the highest standards of scientific accuracy, compliance, and impact. With over 100 peer-reviewed publications in prestigious international journals, TECCRO stands out among the top clinical research organizations in India, providing contract research organization (CRO) services in Mumbai and across India.

The Role of Medical Writing in TECCRO’s Clinical Research Excellence
Ensuring Scientific Rigor and Clarity
TECCRO specializes in translating complex clinical data into clear, precise, and scientifically rigorous documentation. Whether developing clinical trial protocols, drafting clinical study reports (CSRs), or preparing scientific publications, our expert medical writers ensure accuracy and transparency. With over 100 international peer-reviewed publications, TECCRO has built a reputation for excellence in clinical research organizations in Mumbai and beyond.
Streamlining Regulatory Submissions
TECCRO’s medical writing team is well-versed in the preparation of key regulatory documents, including:
Investigator brochures
Clinical study reports (CSRs)
Informed consent forms (ICFs)
Regulatory submissions to DCGI, CDSCO, FDA, and EMA
As a leading contract research organization in Mumbai, we ensure that all documentation complies with global regulatory standards, facilitating smoother approval processes and reinforcing our position among the top 10 clinical research organizations in India.
Facilitating Knowledge Dissemination
With a commitment to scientific advancement, TECCRO actively contributes to the clinical research industry in India through its extensive publication track record. Our publications serve to disseminate valuable research findings, positioning TECCRO among the best clinical research companies in India and a recognized clinical research institute in India.
Ensuring Compliance with Ethical and Scientific Standards
Rooted in Good Clinical Practice (GCP), our medical writing adheres to strict ethical and scientific integrity guidelines. As a trusted clinical research organization in Mumbai, TECCRO ensures that all trial documentation upholds the highest industry standards.
TECCRO’s Comprehensive Approach to Medical Writing
TECCRO offers a full spectrum of medical writing services to support clinical research companies in Mumbai, Ahmedabad, Bangalore, Hyderabad, and Pune, including:
Clinical Trial Protocols: Developing scientifically robust and regulatory-compliant study designs.
Clinical Study Reports (CSRs): Presenting trial results with accuracy and compliance.
Regulatory Submissions: Preparing documentation for CDSCO, DCGI, and international regulatory bodies.
Scientific Publications: Supporting researchers in publishing findings in leading journals, reinforcing TECCRO’s status among top contract research organizations in India.
Timely and Accurate Documentation: A Key to Clinical Research Success
In the fast-paced clinical research industry in India, timely documentation is crucial. TECCRO’s medical writing team ensures efficient delivery of high-quality documentation, keeping trials on track and meeting critical regulatory deadlines.
TECCRO’s Impact on Global Medical Research
TECCRO’s expertise in clinical research services has positioned us among the largest contract research organizations in India. Our experience in preparing regulatory documents, clinical trial protocols, and scientific publications has established TECCRO as a top clinical research organization in Mumbai and a key player in the list of clinical research organizations in India.
With over 100 peer-reviewed publications, TECCRO continues to lead the way in clinical research and medical writing, ensuring that the knowledge generated from our trials contributes to medical advancements worldwide.
Conclusion
As one of the best clinical research companies in India, TECCRO remains dedicated to high-quality research, regulatory compliance, and scientific excellence. Our medical writing services reinforce our reputation as a trusted clinical research organization in Mumbai, ensuring that every document we produce meets the highest standards of accuracy, compliance, and scientific integrity. Whether supporting clinical research companies in Mumbai, contract research organizations in India, or global pharmaceutical and biotech firms, TECCRO continues to set industry benchmarks in medical writing and clinical research.
Would you like us to incorporate specific case studies from TECCRO’s past publications to highlight our success in medical writing?
0 notes
Text
The Importance of Medical Writing Services
In today's rapidly changing healthcare industry, it is more important than ever to convey scientific and medical information accurately and clearly. Medical writing services are crucial for conveying complex medical information in a way that is both understandable and compliant with regulatory requirements. Because they facilitate communication between patients, healthcare professionals, and researchers, these services are critical to the healthcare sector.
What is Medical Writing?
Medical writing is the field of creating legally compliant, scientifically sound texts that convey complex medical and clinical information to a range of readers. It requires a thorough understanding of medicine as well as the ability to simplify and make difficult facts understandable. Whether the information is meant for the general public, medical experts, or regulatory bodies, medical writers ensure that it is reliable and understandable.
Types of Medical Writing Services
A wide number of services are included in medical writing, each specifically designed to address demands in the life sciences and healthcare industries. Among the main kinds are:
Writing Regulations
Because it entails drafting documents that adhere to stringent standards established by regulatory organizations like the FDA, EMA, or other international agencies, regulatory writing is crucial in the healthcare sector. Clinical trial protocols, informed consent forms, and new medication applications are examples of these papers. Since any oversight might postpone clinical trials or medication approval, regulatory writers must make sure that all documents adhere to regulatory regulations.
Writing in Science and Medicine
The creation of clinical research-related papers, including research articles, clinical study reports (CSRs), and manuscripts for medical journals, is the main goal of scientific and clinical writing. This kind of writing guarantees that study results are correctly reported and presented, enabling the data to be used by the larger medical community.
Communications and Marketing for Medicine
Writing for medical marketing entails producing promotional material that complies with legal and ethical requirements. Brochures, websites, ads, and other materials used to convey the advantages of medical goods or services might fall under this category. Medical marketing content must strike a balance between truth and promotion, making sure that all claims are backed by science.
Writing for Medical Education
Writing for medical education includes producing instructional materials, patient information tools, and continuing medical education (CME) programs. By disseminating current information and supporting patients' and healthcare professionals' ongoing professional growth, this kind of writing is essential to enhancing healthcare outcomes.
Why Medical Writing Services Are Essential
For a number of reasons, including guaranteeing precision, adherence to regulations, and efficient communication within the healthcare industry, professional medical writing services are essential.
Maintaining Precision and Adherence
Because even small mistakes can have major repercussions in the healthcare sector, medical writing demands accuracy. Accurate communication of complicated clinical material requires medical writers to comprehend it. In order to assist pharmaceutical businesses, healthcare providers, and researchers in navigating the complex regulatory environment, they must also make sure that all papers adhere to the most recent regulatory rules.
Bridging the Gap Between Science and the Public
By converting intricate medical research into easily understood English, medical writers contribute to closing the gap between the general public and the scientific community. This is particularly crucial for informing patients and non-experts about new medical procedures, research results, or health advice so they may make educated decisions regarding their health.
The Impact of Medical Writing on Healthcare
Beyond only creating documentation, medical writing services have a significant impact on improving patient outcomes, furthering medical research, and strengthening healthcare procedures.
Promoting Innovation and Research in Medicine
Medical writers contribute to the acceleration of medical innovation through research articles, clinical reports, and regulatory publications. Medical writing directly contributes to the quicker release of novel medications and treatments onto the market, which eventually benefits patients and enhances healthcare by ensuring that clinical trial data is conveyed correctly.
Enhancing the Results for Patients
Another area where medical writing is useful is in patient education materials. Patients can better manage their health and comprehend their illnesses and treatment choices when they read clear, well-written literature. Gaining more knowledge improves decision-making and health results, which benefits patients' general wellbeing.
Conclusion: The Future of Medical Writing Services
The need for qualified medical writing services will only increase as the healthcare sector changes due to new laws, research, and technology developments. More than ever, medical writers play a critical role in helping different stakeholders understand complicated material. Purchasing top-notch medical writing services helps advance medical research and enhance patient care in addition to guaranteeing the correctness and compliance of medical material.
0 notes
Text
Clinfinite Solutions Advances Medical Writing for Healthcare Professionals.

1. Introduction
In today's fast-evolving healthcare and pharmaceutical landscape, the need for accurate, clear, and well-structured medical documents has never been more critical. Medical writing services play a pivotal role in bridging the gap between complex scientific information and its intended audience, ensuring effective communication that supports advancements in healthcare. Whether for regulatory submissions, scientific publications, or patient education materials, medical writing is essential for delivering impactful and precise messages.
2. Types of Medical Writing Services
Medical writing services cover a wide spectrum of documents tailored to meet the diverse needs of the medical and scientific community.
Regulatory Writing: This includes clinical trial protocols, investigator brochures, and regulatory submissions required for drug approvals. These documents must adhere to strict regulatory standards and guidelines.
Scientific Publications: Manuscripts, journal articles, conference abstracts, and posters fall under this category, helping researchers share their findings with the broader scientific community.
Medical Marketing Content: Sales aids, brochures, white papers, and product monographs aim to engage healthcare professionals and promote pharmaceutical products effectively.
Educational Material: Patient leaflets, e-learning modules, and continuing medical education (CME) content provide valuable information to patients and healthcare providers, enhancing understanding and compliance.
3. Why Are Medical Writing Services Essential
High-quality medical writing is essential for ensuring clarity, compliance, and credibility in medical and scientific communications.
Clarity: Simplifying complex medical data for diverse audiences, from regulatory agencies to patients.
Compliance: Adhering to regulatory standards like ICH, FDA, and EMA guidelines to ensure documents meet industry expectations.
Credibility: Presenting accurate, evidence-based information to build trust among stakeholders, including clinicians, patients, and researchers.
4. The Process of Medical Writing
Crafting a high-quality medical document requires a meticulous and collaborative approach.
Understanding Requirements: The process begins with a clear understanding of the client’s goals, the target audience, and the specific document type.
Research and Data Gathering: Medical writers collect accurate data from reliable sources, including clinical studies, scientific literature, and regulatory databases.
Drafting and Editing: The next step involves structuring the content, drafting, and revising to ensure scientific accuracy and clarity.
Review and Quality Assurance: Finally, documents undergo rigorous review for compliance, consistency, and quality before submission or publication.
5. Skills Required in Medical Writing
Successful medical writers combine scientific expertise with exceptional communication skills.
Scientific Knowledge: A deep understanding of medical and scientific terminology is crucial.
Writing Expertise: The ability to write clearly, concisely, and persuasively for different audiences.
Regulatory Awareness: Familiarity with international guidelines such as ICH, FDA, and EMA is essential.
Attention to Detail: Precision is critical when interpreting complex data and ensuring consistency in documents.
6. Benefits of Outsourcing Medical Writing Services
Partnering with professional medical writing service providers can significantly enhance the quality and efficiency of your projects.
Expertise: Access to specialized knowledge across various therapeutic areas.
Efficiency: Saves time and resources, allowing organizations to focus on core activities.
Compliance: Ensures adherence to regulatory requirements, reducing the risk of errors and delays.
Scalability: Allows organizations to handle projects of varying sizes and complexities with ease.
7. Choosing the Right Medical Writing Service Provider
Selecting the right medical writing partner is key to achieving your project’s goals.
Expertise and Experience: Look for a team with proven expertise in your therapeutic area.
Regulatory Knowledge: Ensure the provider is well-versed in current guidelines and standards.
Portfolio and References: Evaluate their previous work and seek client testimonials.
Timeliness and Quality: Choose a partner known for meeting deadlines without compromising on quality.
8. Trends and Challenges in Medical Writing
As the medical field evolves, so do the trends and challenges faced by medical writers.
Emerging Trends: The integration of artificial intelligence (AI) in medical writing, a growing focus on patient-centric documents, and the rise of digital healthcare content.
Challenges: Staying updated with regulatory changes, interpreting complex data, and addressing the needs of a global audience with diverse cultural and linguistic backgrounds.
9. Conclusion:
Medical writing services play a pivotal role in bridging science and communication, ultimately driving advancements in healthcare and patient outcomes. By delivering accurate, clear, and well-structured documents, these services support innovation, compliance, and collaboration across the medical and scientific community. Organizations investing in professional medical writing services can ensure their projects’ success while contributing to the broader goal of improving global healthcare.
Get more information here .....
0 notes
Text
#protocol writing in clinical research#protocol writing for acne vulgaris#writing an acne protocol#acne clinical research protocol writing#clinical trial protocol writing#drafting protocol for clinical trial#writing protocols for clinical trials#clinical trial protocol example#phase 1 clinical trial protocol template
0 notes
Text
Document Management System for Clinical Trials

In the realm of medical research, the efficiency and accuracy of managing documents are paramount to the success of clinical trials. Document Management Systems (DMS) have emerged as indispensable tools, streamlining the complex process of organizing, storing, and retrieving critical information integral to clinical trials. These systems are designed to address the unique challenges faced in the healthcare industry, ensuring compliance, security, and accessibility of essential trial documents.
Clinical trials entail a labyrinth of paperwork, from protocol outlines and informed consent forms to patient records and regulatory submissions. The sheer volume of documentation demands meticulous organization and stringent oversight. A robust clinical trial document management system acts as a centralized hub, consolidating diverse documents while maintaining version control, facilitating collaboration, and ensuring adherence to stringent regulatory standards such as Good Clinical Practice (GCP) guidelines.
Evolving Technologies and Future Trends
The evolution of DMS in clinical trials continues to be fueled by technological advancements. Artificial Intelligence (AI) and Machine Learning (ML) algorithms are increasingly integrated into DMS, offering predictive analytics to forecast potential risks or bottlenecks in document management. These technologies automate document classification, extraction, and analysis, enhancing efficiency and decision-making processes.
Moreover, the emergence of blockchain technology holds promise for enhancing the security and immutability of clinical trial data. Blockchain-based DMS can provide an incorruptible ledger, ensuring tamper-proof documentation and transparent audit trails, thus bolstering trust among stakeholders.
Addressing Data Privacy and Security
With the growing concern over data breaches and cyber threats, ensuring robust data privacy and security measures within document management system for clinical trials is imperative. Encryption protocols, multi-factor authentication, and role-based access control are essential features to safeguard sensitive patient information and maintain compliance with data protection regulations like HIPAA (Health Insurance Portability and Accountability Act) and GDPR (General Data Protection Regulation).
Adoption Challenges and Strategies
While the benefits of DMS in clinical trials are evident, adoption challenges persist. Resistance to digital transformation, budget constraints, and concerns regarding data security often impede widespread implementation. To overcome these hurdles, comprehensive change management strategies coupled with user-centric designs are essential. Engaging stakeholders early in the selection and implementation phases, along with tailored training programs, can promote a smoother transition and maximize user acceptance.
Regulatory Compliance and Standardization
Regulatory bodies continue to refine and update guidelines concerning document management in clinical trials. DMS providers must stay abreast of these evolving regulations to ensure their systems remain compliant. Standardization efforts, such as the adoption of industry-wide metadata standards and interoperability frameworks, facilitate seamless data exchange and collaboration among different stakeholders and systems.
One of the pivotal features of a DMS tailored for clinical trials is its capability to support the entire lifecycle of documents. From the initial drafting of protocols to the final submission of reports, these systems track and manage each document's progression. Version control mechanisms within the DMS prevent errors resulting from outdated or conflicting information, ensuring that all stakeholders access the most current data.
Moreover, compliance with regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) is non-negotiable in clinical research. Document Management Systems equipped with audit trails and security protocols ensure traceability and data integrity, aligning with stringent compliance requirements. This fosters transparency and accountability while safeguarding against unauthorized access or alterations to sensitive trial information.
Efficiency in document retrieval is another crucial aspect of a DMS. Researchers, clinicians, and regulatory authorities often require swift access to specific documents. Advanced search functionalities and categorization systems implemented in these systems expedite the retrieval process, saving valuable time and enhancing productivity.
The collaborative nature of clinical trials necessitates seamless communication and sharing of documents among multiple stakeholders dispersed across different geographical locations. Cloud-based DMS platforms offer real-time accessibility, enabling simultaneous access and collaboration while maintaining data security. This facilitates interdisciplinary teamwork, allowing researchers, clinicians, and sponsors to contribute and review documents efficiently.
Furthermore, the integration of electronic signatures and workflows within DMS platforms streamlines the approval processes for various documents. Electronic signatures, compliant with regulatory standards, expedite approvals, reducing the reliance on cumbersome paper-based workflows and minimizing the risk of errors or delays.
Despite the myriad advantages offered by Document Management Systems, challenges persist. Implementation and adoption of these systems require robust training programs to familiarize users with the platform's functionalities. Resistance to change, especially in traditionally paper-based environments, may hinder the seamless integration and utilization of DMS.
In Summation
The future of clinical trials hinges significantly on the efficacy and sophistication of Document Management Systems. These systems transcend mere document storage; they are pivotal in driving efficiency, transparency, and collaboration across the clinical trial lifecycle. The continuous integration of innovative technologies, stringent adherence to regulatory standards, and concerted efforts to address adoption challenges will further propel the evolution and widespread adoption of advanced edocs document management systems in revolutionizing the landscape of clinical research. Ultimately, this progression will pave the way for more expedited, reliable, and patient-centric healthcare advancements.
In conclusion, Document Management Systems tailored for clinical trials play an instrumental role in revolutionizing the documentation landscape within the healthcare and research sectors. These systems alleviate the burdens associated with document organization, compliance, and accessibility, thereby fostering efficient, secure, and compliant management of essential trial documents.
Embracing innovative DMS technologies is pivotal in advancing the trajectory of clinical research, promoting transparency, collaboration, and ultimately, better patient outcomes. Want to know more about how Octalsoft can help you with document management for your next clinical trial? Book a demo with us now!
0 notes
Text
Labcorp Clinical Report Writer Coordinator Job Vacancies Labcorp, a global leader in life sciences and healthcare solutions, is hiring a Clinical Report Writer Coordinator in Bangalore, India. This entry-level position offers an exciting opportunity to develop expertise in clinical trial documentation, working within a dynamic team environment. If you have a passion for clinical writing and are ready to kick-start your career, this role is for you! Labcorp Hiring Clinical Report Writer Coordinator Labcorp is actively seeking a dedicated and detail-oriented individual to join their LMVRS (Labcorp Medical Writing and Reporting Services) team. As a Clinical Report Writer Coordinator, you will contribute to producing high-quality clinical trial documentation that supports research and innovation in healthcare. Key Job Details Job Title: Clinical Report Writer Coordinator I Category: Clinical Location: Bangalore, India Job ID: 2444315 Job Type: Full-Time Job Responsibilities As a Clinical Report Writer Coordinator, you will: The Report Writer Coordinator is an entry level position in the LMVRS group, learning the Labcorp writing standard and learning to draft and edit reports to promote product consistency. Learns to use software tools to efficiently and accurately complete job duties. Learns and understands any new/changed conventions or standard language. Creates report folders and requests all needed information, documentation and any other relevant information from the respective departments. Creates part of the initial draft of lower complexity clinical trials internal or client facing documents using pre-defined templates or client-supplied information with guidance from more senior colleagues and using study protocol information, SOPs and/or lab data. Creates data tables, as needed, using the corresponding database(s). Performs review and quality control (QC) as applicable. Edits internal or client facing documents, as needed, after review, ensuring that company convention, style, format and terminology have been used. Independently utilizes workload management tool to track and document data and report completion activities. Ensures compliance to applicable departmental Standard Operating Procedures and Work Instructions. Maintains/develops training manuals. Effectively manages own time and deadlines. Effectively manages task status tracking and time recording. May provide backup assistance to colleagues, as appropriate. Performs other duties as assigned. [caption id="attachment_111808" align="aligncenter" width="1200"] Labcorp Hiring Clinical Report Writer Coordinator – Entry-Level Opportunity in Bangalore[/caption] Eligibility Criteria To apply for this role, candidates must possess: Strong writing and communication skills. Proficiency in using software tools for data management and document creation. Excellent time management abilities. This position is ideal for individuals eager to grow in the field of clinical writing within a supportive and innovative organization. How to Apply To apply for the Clinical Report Writer Coordinator role, visit the official Labcorp careers page at the link below: Apply Here Equal Opportunity Employer Statement Labcorp is proud to be an Equal Opportunity Employer. Employment decisions are based on qualifications and business needs, without discrimination based on race, gender, religion, or other protected characteristics.
0 notes
Text
Clinical Trials Support Services: Enabling Medical Advancements through Quality Research

Types of Clinical Trials Support Services Clinical trial support encompasses a wide array of services required to conduct medical research efficiently and effectively. Some of the key types of support services include: Regulatory Support
Clinical trials must adhere to stringent regulations and standards to ensure safety and ethical conduct. Regulatory support services help navigate these requirements and secure necessary approvals. Regulatory experts aid with preparation of documents such as clinical trial applications and maintaining compliance throughout the trial lifecycle. Patient Recruitment Support
Finding suitable human volunteers is one of the biggest challenges in clinical research. Dedicated patient recruitment teams leverage different online and offline strategies to promote awareness of trials and screen potential candidates as per eligibility criteria. Their efforts are vital for timely patient enrollment and study completion. Site Management Support
Managing the operational aspects at Clinical Trials Support Services sites spread across locations requires dedicated coordination. Site management services take care of site initiation activities, training investigators and staff, addressing their queries, facilitating logistics and ensuring protocol adherence. This helps sites function efficiently and focus on participant care. Biostatistics and Data Management Support
Clinical trials generate huge volumes of data at each stage that needs to be captured, assessed and reported as per quality standards. Biostatisticians and clinical data managers employ their analytical skills and use specialized software to plan the data collection methodology, perform interim analyses, and compile the clinical study report. Medical Writing Support
From drafting patient consent forms and recruitment material to compiling clinical study reports – medical writing plays a significant role in clearly communicating critical information for different stakeholders. Experienced medical writers utilize their medical and regulatory expertise to develop high-quality documentation tailored to the audience. Safety Monitoring and Pharmacovigilance
Ensuring participant safety is the utmost priority in clinical research. Independent safety boards and pharmacovigilance teams closely monitor trials for any adverse events. They analyze trends, determine causality and take necessary actions to minimize risks to human subjects. Logistics Support
Timely shipment of investigational products, medical supplies and equipment to sites spread across multiple countries requires efficient logistics management. Logistics coordinators arrange for customized solutions like GPS-enabled transportation, proper storage facilities and distribution tracking systems. Advantages of Outsourcing Clinical Trial Support Services With the complexity of modern clinical trials, most pharmaceutical and biotech organizations leverage specialized clinical research organizations (CROs) to outsource support functions: Access to Expertise
CROs employ multidisciplinary teams of highly qualified clinical research experts with vast international experience. Their combined and focused skillsets can deliver superior services than an in-house function. Cost Savings
Outsourcing non-core operations frees up internal resources for other strategic activities. It also offers scalability with pay-as-you-go fee-for-service models to match financing needs at each stage. This provides significant cost advantages over building in-house infrastructure. Infrastructure and Technology
CROs make large investments in state-of-the-art technologies, facilities and resources required to support global clinical trials. Outsourcing leverages these resources that would otherwise require high capital expenditure for sponsors. Resource Flexibility
CRO staffing can easily scale up or down based on changing study requirements without long-term commitments. This flexibility enables sponsors to focus on core development work while managing variable external support needs. Compliance Expertise
With experience spanning hundreds of trials globally, CROs have in-depth knowledge and polished processes to ensure compliance with regulations in different regions. This mitigates risks of non-compliance for sponsors. Conducting Large Multinational Trials
Some clinical programs involve complex trials across dozens of countries simultaneously. Few sponsors have the bandwidth to internally coordinate such large-scale global operations. CROs specialize in seamlessly executing multinational clinical programs. Quality Clinical Trial Support Services Define Clinical Research Success A few key aspects guarantee the delivery of high-quality clinical trial support services: Extensive Therapeutic Experience
CROs with proven track record of supporting various therapeutic areas can adeptly cater to specific sponsor requirements and anticipate challenges through their past learning. Robust Quality Management Systems
Adopting global quality standards like GCP, ISO certified processes and ongoing audits ensure consistent adherence to protocol, timely issue resolution and generation of reliable data. Technology Integrated Solutions
Leveraging customized applications for functions like patient recruitment, site payments, interactive drug supply chain tracking and integrated clinical data capture enhances efficiency. Prequalified Global Network
A pre-established pool of qualified clinical sites, laboratories and investigators across regions facilitates rapid study startup. Their pre-qualification saves time in site feasibility assessments. Proactive Communication Culture
Regular sponsor interactions, performance reporting and swift issue escalation enable identifying risks early and collaborative troubleshooting. This drives seamless collaboration. Clinical Trials Support Services Talent Development Focus
Ongoing training and skill development programs for both sponsor and CRO staff keep the clinical research talent abreast with evolving science and best practices to deliver best quality.
Get more insights on Clinical Trials Support Services
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement.
(LinkedIn- https://www.linkedin.com/in/priya-pandey-8417a8173/)
#Clinical Trials Support Services#Medical Research#Trial Management#Clinical Study#Patient Recruitment#Data Management#Regulatory Compliance
0 notes
Text
Streamlining the Afghanistan Drug Registration: A Comprehensive Guide for Approval and Compliance
MoH: Ministry of Public Health
MoH website: https://moph.gov.af/en
Regulatory Authority: Afghanistan Food and Drug Authority National Medicine and Health Products Regulatory Authority (NMHRA)
Link for Regulatory Authority: https://afda.gov.af/index.php/en/market-0
Afghanistan Drug Registration Procedure
Pre-Market Afghanistan Drug Registration Procedure
In the ever-changing healthcare landscape of Afghanistan, it is critical to effectively regulate medical goods and services. This paper explores the complex pre-marketing processes, including planning and policy, licensing, marketing permits, supply, price, and the vital oversight and management of clinical trials.
1. Policy & Plan
Planning and Coordination is the first step towards a strong regulatory system. The Ministry of Public Health in Afghanistan assumes a leading role in coordinating the efforts of many parties participating in the pre-marketing phase. Manufacturers, scientists, medical experts, and governmental organizations fall under this category. The foundation for efficient cooperation and communication is laid by strategic planning.
The success of pre-marketing operations depends heavily on clear and efficient communication. It is necessary to keep stakeholders updated on guidelines, regulations, and policy changes. Clear and frequent communication channels are set up to provide manufacturers, distributors, and other pertinent parties with information.
Information is a key component of regulatory decision-making, according to information management and evaluation (M&E). Strong data management systems make sure that data is gathered, examined, and assessed about clinical trials, healthcare goods, and market dynamics. Mechanisms for routine monitoring and assessment are in place to determine where improvements might be made and to gauge the success of current policies.
The foundation of healthcare regulation is the drafting and revision of legislative documents. Lawmakers in Afghanistan create and amend laws on a regular basis to meet changing needs and conform to global norms. Carefully constructed legislative documents handle licensing, permits, cost, and clinical study regulation.
2. Licensing
This essential pre-marketing step makes sure that only safe and efficient medical supplies reach consumers. A rigorous vetting process is required of businesses looking to sell their goods in Afghanistan. Complete information provided during the licensing application is used by the regulatory body to evaluate the product’s quality, safety, and efficacy.
3. Permission to Price and Supply the Market (Valuation)
Supply Chain Oversight: Businesses need permits to sell their goods to keep a safe and effective supply chain. By ensuring that products are supplied and distributed in accordance with regulatory criteria, this reduces the possibility that inferior or counterfeit goods may enter the market.
Pricing and Valuation: Accessible healthcare is facilitated by the establishment of reasonable pricing structures and the appraisal of healthcare items. To establish fair prices, regulatory agencies collaborate with manufacturers, considering aspects including quality, production costs, and market conditions. The goal of this procedure is to achieve a balance between sustainability and affordability.
4. Control and Regulation of Clinical Research
Clinical research is essential to expanding our understanding of medicine and guaranteeing the security and effectiveness of medical treatments.
To safeguard participants and maintain ethical standards, clinical research are tightly regulated and controlled by Afghan regulatory organizations. This method includes constant monitoring, informed consent processes, and a close examination of study protocols.
Post-Market Afghanistan Drug Registration Procedure
Beyond the original drug registration procedure, there is a continuous commitment to ensuring the safety, efficacy, and quality of pharmaceuticals.
1. Examining and Implementing Laws and Rules
Supervisory Authority: Afghanistan passionately believes that post-market inspections are essential to upholding pharmaceutical standards. To make sure that manufacturing sites, distribution networks, and retail locations are adhering to laws and regulations, regulatory bodies regularly audit them. The goal of this thorough inspection is to find and address any deviations from accepted norms.
Cooperation with Stakeholders: Afghan regulatory agencies work closely with pharmaceutical companies, medical practitioners, and other stakeholders to improve post-market surveillance. This cooperative strategy encourages a shared accountability for maintaining pharmaceutical standards, with continual education and communication to address issues and encourage adherence.
2. Pharmacovigilance, or Market Surveillance
Pharmacovigilance Framework: An essential part of Afghanistan’s post-market processes is market surveillance via pharmacovigilance. Data on the safety of pharmaceutical products are systematically collected, analysed, and interpreted as part of the pharmacovigilance framework. This continuous procedure aids in identifying and evaluating side effects or any other issues relating to drugs.
Adverse Event Reporting: It is crucial for patients, healthcare providers, and pharmaceutical companies to report adverse occurrences connected to their products. There is a strong reporting mechanism in place that makes it possible to promptly submit data on unanticipated side effects or other safety issues. To decide what steps to take next, regulatory bodies thoroughly look at these reports.
Risk management and signal detection: One aspect of pharmacovigilance work is the ongoing observation of signals that can point to safety issues. Regulatory agencies strive to identify new dangers and proactively address them. This could entail changing the product’s label, releasing safety alerts, or, in the worst situations, taking the medication off the market.
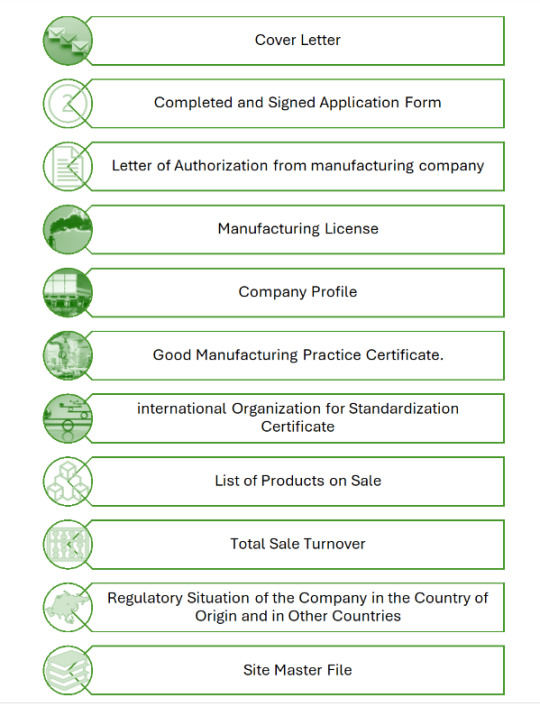
Documents Required for Product Registration in Afghanistan
1. Official introduction-letter and addresses of the company issued by the company itself.
2. License of producing medicine and medical equipment and certification of three authorized organizations of the origin country and that of WHO.
3. Special license for producing specific items from MoPH and certifications from high authorities of the origin country and that of WHO.
4. License of exportation.
5. Documents for the usage of the products inside the origin country.
6. Quality control documents of the company and also quality control documents from the central laboratory and ministry of MoPH of the origin country.
7. Specific documents of the medicine such as: list of formulation including standard formulation of the medicine, list of prices including reasonable prices, packing list with the specification about packing material, and method of packing. Procedure for controlling the production process, specification of its contents, documents of analysis, method of analysis, reliability of the method of analysis certification of the contents and pharmacological report of the medicine.
8. Original stamp of the company on the relevant documents.
9. Certification of the ministry of MoPH and stamp of chambers of commerce of the origin country and issuance of them through trading department of the embassy of Afghanistan located on the producing country.
10. Analysis the sample of the medicine in the general medicine analyzing laboratories of Afghanistan and registering the mentioned products.
Registration Requirements for Product Registration in Afghanistan
Fees for Afghanistan Drug Registration
AFN (50,000)
License Validity for Afghanistan Drug Registration
3 years.
Local Authorized Representative
Yes, a local Authorized Representative is required before you place your product on the market.
Originally Published at: https://omcmedical.com/afghanistan-drug-registration-procedure/
0 notes
Text
Roles of Medical Monitor and Medical Writing in Clinical Research
In the realm of clinical research, the roles of medical monitors and medical writing are indispensable, serving as crucial pillars in the development and execution of clinical trials. While distinct in their functions, these roles work in tandem to ensure the safety, efficacy, and compliance of investigational treatments. Let's delve into the intricacies of medical monitoring and medical writing, exploring their significance, responsibilities, and contributions to advancing medical science.
Medical Monitor: Safeguarding Participant Safety and Study Integrity
The medical monitor plays a pivotal role in clinical trials, serving as the principal medical expert responsible for overseeing the safety and welfare of study participants. This role requires a deep understanding of the study protocol, investigational product, and relevant therapeutic area. The medical monitor collaborates closely with the clinical research team to ensure adherence to ethical standards, regulatory requirements, and good clinical practice (GCP) guidelines.
Key responsibilities of the medical monitor include:
Protocol Review and Approval: Reviewing and providing input on the study protocol to ensure scientific rigor, participant safety, and regulatory compliance.
Safety Oversight: Monitoring participant safety throughout the duration of the trial, including reviewing adverse events, protocol deviations, and serious adverse events (SAEs).
Data Review and Analysis: Analyzing study data to assess safety, efficacy, and overall study integrity, identifying trends or potential concerns that may require further investigation.
Investigator Training and Support: Providing guidance and support to investigators and site staff regarding protocol adherence, safety reporting, and study conduct.
Regulatory Compliance: Ensuring compliance with regulatory requirements and reporting obligations, including timely submission of safety reports to regulatory authorities.
Medical Writing: Communicating Science with Clarity and Precision
Medical writing plays a crucial role in translating complex scientific data and research findings into clear, accurate, and regulatory-compliant documents. This role encompasses a wide range of activities, including the preparation of clinical study protocols, informed consent forms, clinical study reports (CSRs), regulatory submissions, and scientific publications. Medical writers collaborate closely with cross-functional teams, including clinical research, regulatory affairs, and biostatistics, to ensure the accuracy and integrity of written documents.
Key responsibilities of medical writing include:
Protocol Development: Assisting in the development and writing of clinical study protocols, ensuring alignment with regulatory requirements and scientific standards.
Informed Consent Form (ICF) Preparation: Drafting clear and comprehensive ICFs that provide study participants with essential information about the trial, including risks, benefits, and study procedures.
Clinical Study Report (CSR) Writing: Compiling and synthesizing study data into CSRs, which summarize the study design, methodology, results, and conclusions in a comprehensive and scientifically sound manner.
Regulatory Submissions: Supporting the preparation and submission of regulatory documents, including Investigational New Drug (IND) applications, New Drug Applications (NDAs), and Marketing Authorization Applications (MAAs).
Scientific Publications: Collaborating with investigators and key opinion leaders to prepare manuscripts for publication in peer-reviewed journals, disseminating study findings to the scientific community.
The Synergy Between Medical Monitor and Medical Writing
While distinct in their functions, the roles of medical monitoring and medical writing are interconnected and complementary. The medical monitor relies on the expertise and support of medical writers to communicate study findings, safety data, and regulatory submissions effectively. Conversely, medical writers depend on the guidance and input of medical monitors to ensure the accuracy and scientific integrity of written documents.
The synergy between medical monitoring and medical writing is essential for the successful execution of clinical trials and the advancement of medical science. By working collaboratively and leveraging their respective expertise, these roles contribute to the development of safe and effective treatments, ultimately benefiting patients and improving healthcare outcomes.
In conclusion, the roles of medical monitors and medical writing are indispensable in the field of clinical research. While the medical monitor safeguards participant safety and study integrity, the medical writer communicates scientific data and findings with clarity and precision. Together, these roles play a vital role in advancing medical science and bringing innovative treatments to patients in need.
0 notes
Text
What are physician writing services, and how can they benefit medical professionals?

Physician writing services encompass a range of professional writing and editing solutions tailored specifically for medical professionals, including doctors, researchers, and healthcare organizations. These services are designed to assist with various written materials crucial to the medical field, such as research papers, clinical trial reports, medical marketing content, regulatory documents, and patient education materials.
Non-profit global Medical Affairs organizations play a vital role in advancing healthcare by facilitating collaboration, promoting education, and driving innovation within the medical community. Professional medical organizations, on the other hand, bring together experts from various specialties to exchange knowledge, develop guidelines, and advocate for best practices in patient care.
Global medical development involves the process of researching, testing, and launching new medical products and treatments on a global scale, with a focus on improving patient outcomes and addressing unmet medical needs worldwide.
Physician writing services offer several key benefits to medical professionals and organizations:
Expertise and Accuracy: These services are provided by experienced medical writers who possess in-depth knowledge of medical terminology, research methodologies, and regulatory requirements. They ensure that written materials are accurate, scientifically sound, and compliant with industry standards.
Time Savings: Medical professionals often have demanding schedules, leaving limited time for writing and editing tasks. Physician writing services can save time by handling these responsibilities efficiently, allowing healthcare professionals to focus on patient care, research, and other critical activities.
Quality Assurance: By leveraging the expertise of professional medical writers, organizations can maintain a high standard of quality across their written communications. This is particularly important for regulatory documents, where accuracy and clarity are paramount.
Compliance and Regulatory Support: Medical writing services can assist with the preparation of regulatory submissions, ensuring that documentation meets the stringent requirements of regulatory authorities. This support streamlines the approval process for new medical products and treatments.
Enhanced Communication: Clear and engaging communication is essential in healthcare. Physician writing services help convey complex medical information in a concise and accessible manner, improving communication with patients, colleagues, and stakeholders.
Publication and Presentation Support: Medical writers can assist with preparing manuscripts for publication in scientific journals and creating compelling presentations for conferences and meetings. This support enhances the visibility and impact of research findings.
Tailored Solutions: Physician writing services can be customized to meet specific needs, whether it’s developing educational materials for patients, creating marketing content for medical devices, or drafting clinical trial protocols.
In summary, physician writing services offered by professional organizations play a crucial role in supporting global medical development by ensuring accurate, compliant, and impactful communication within the healthcare industry. These services enable medical professionals to navigate complex writing tasks more efficiently while maintaining a focus on advancing patient care and medical research.
To Know More Visit: https://medicalaffairs.org/
0 notes
Text
What is Post-Market Clinical Follow-Up?
PMCF study is carried out for CE certified medical devices that are placed in the market. It’s a method of proactive collection of clinical data to analyze the emergent risks/side-effects of the medical device to demonstrate the safety and performance as per the intended purpose, throughout the expected lifetime of the device.
Performance and safety parameters are predefined with acceptance criteria based on the benchmark devices data, and milestones are designed for several PMCF studies and activities. The literature screening and registries study also supports PMCF findings. The sample size is predefined as per the sales of the device to study the emergent risks. Benefit-risk ratio analysis is performed as per the PMCF findings, for the acceptability of the device in the market. The PMCF findings are also documented in Technical Documentation, Risk File, SSCP, CER and PSUR
PMCF Includes
PMCF Procedure
PMCF Plan
PMCF Report
PMCF Plan
PMCF Protocol
Sample Size
Design of Milestones with follow-up period
Design of measurable endpoints for performance and safety
Ethical Committee approval
Questionnaire/Survey form
Patient/subjects consent form
Clinical Investigation/Trial procedure (if any)
Real World Evidence
Statistical Significance
PMCF Report
Milestones completion
Real World Evidence analysis
Literature Screening
Registries
Questionnaires and Surveys results
Feedback from users/end user
Clinical Investigations/Trials results (if planned any)
Outcome of performance and safety endpoints
Risk benefit analysis
Residual Risks (if any)
Statistical Analysis
CER citation
Frequency of PMCF update
IZiel Healthcare offers PMS services to our clients where we perform end to end Post Market Surveillance activities. These services can be PMS Plan, PMS/PSUR Reports, PMCF Plan and Reports, Trend Reporting, Complaint Handling, Clinical Evaluation, Risk Benefit Management and many more.
We have expertise in designing PMCF Plan and drafting reports for our clients for all the Classes of Medical devices – Class IIa, Class IIb and Class III.
You may contact us at Contact Us – Iziel.
0 notes